ICL (implantable contact lens) or PIOL (phakic intraocular lens)
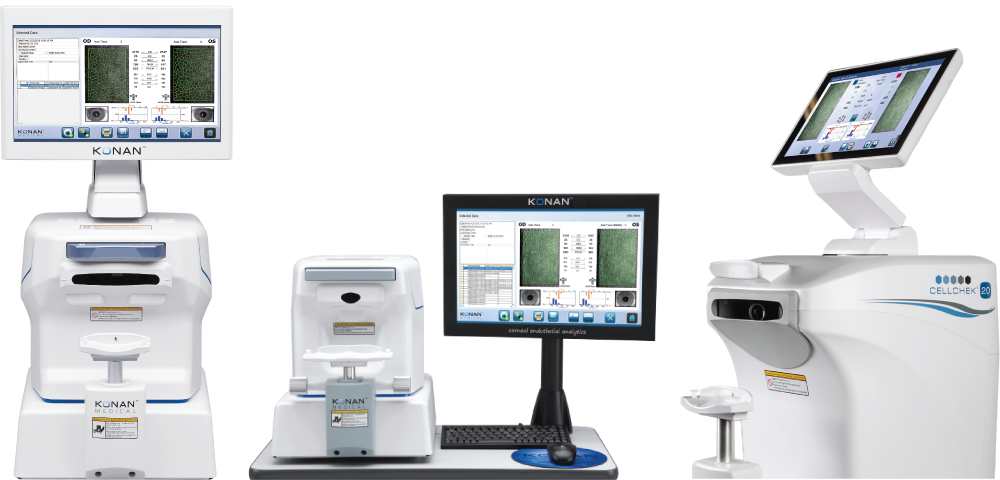
An endothelial cell count to verify that a patient’s cornea has an adequate endothelial cell density is an FDA labeling requirement for patient screening and selection prior to implantation of phakic intraocular lenses (Intraocular Contact Lenses, or “ICLs”). Without an adequate endothelial cell count, the current ICLs are contraindicated for implantation and clearly highlights the importance of this screening procedure. Endothelial cell density must be accurately checked preoperatively to ensure that a patient is a suitable candidate, and then should be monitored periodically postoperatively to verify the endothelium’s continued health. Although an endothelial cell count can be crudely estimated using a card developed in the 1980′s, this technique has significant limitations that are now well answered with Konan’s specular microscopy technologies:
-
High precision and repeatability
-
Excellent visualization of even stage +1 / +2 guttata that could significantly skew results
-
Analysis of both cell counts and cell morphology changes (morphology is not assessed with a “card”)
-
Changes in morphology can be a very sensitive indicator of corneal stress
-
Unique ability to identify endothelial data location and assess trends over time
-
Acquiring endothelial cell count or morphology changes over time from dissimilar locations can present erroneous assumptions on trends analysis
-
Technician administered diagnostic test with full photographic documentation and statistics in only seconds.
-
Does not require additional physician chair time and eliminates the inherent problems of “card” estimates.
-
Important complementary uses for pre-operative assessments of corneal and other anterior segment procedures

Theodore Perl, MD
It is important to select patients whose endothelial cell density and morphology are normal.
Avoiding Complications with Phakic IOLs” (Cataract and Refractive Surgery Today, October 2009)
ICL Products
Premium refractive products require premium attention to eligibility. Konan CellChek provides easy to use yet robust analytics to assure proper patient selection. There is a reason major manufacturers of ICL’s use Konan specular microscopes for collection of FDA safety data. Your patients deserve no less.
FDA Professional labeling including ECD minimum requirements:
FDA Professional labeling including ECD minimum requirements:
Visian ICL, Staar Surgical, Verisyse, Abbot Medical Optics, AcrySof Cachet, and ALCON are trademarks of their respective owners.
Scleral Lenses with High Risk Endothelial Cell Density
Patients with low endothelial cell densities may benefit from specular microscopy assessment to verify adequate density prior to fitting a scleral lens and to monitor continued supportive endothelial cell architecture if scleral lens treatments are implemented. Many experts recommend minimum cell densities of 800 to 1,000 cell / mm² to support scleral lenses.
Fundamentals of the importance of endothelial cell density to support implantation of phakic IOLs described above, may have parallels in decision making for scleral lens therapies.

Christine Sindt, OD, FAAO
Clinical Professor Ophtalmology and Vision Sciences, University of
Iowa
It is common to experience corneal edema with endothelial
cell counts under 800 cells/mm².
Navigating the Slippery Slope (Review of Optometry, April 2015)

Eef van der Worp, BOptom, PhD, FAAO, FIACLE, FBCLE, FSLS
Pacific University, Oregon | University of Maastricht, Netherlands
Endothelial cell count of less than 800 cells/mm² is where the
problems may arise (Sindt 2010a), and endothelial cell counts
<1,000 cells/mm² should be handled with extra care and should not be fitted with scleral lenses to avoid edema.