News from Konan
Blazing fast, one-touch examination for both eyes, CellChek 20 sets the new bar for user friendly, high quality corneal endothelial imaging and analytics.
Irvine, CA – September 30, 2020
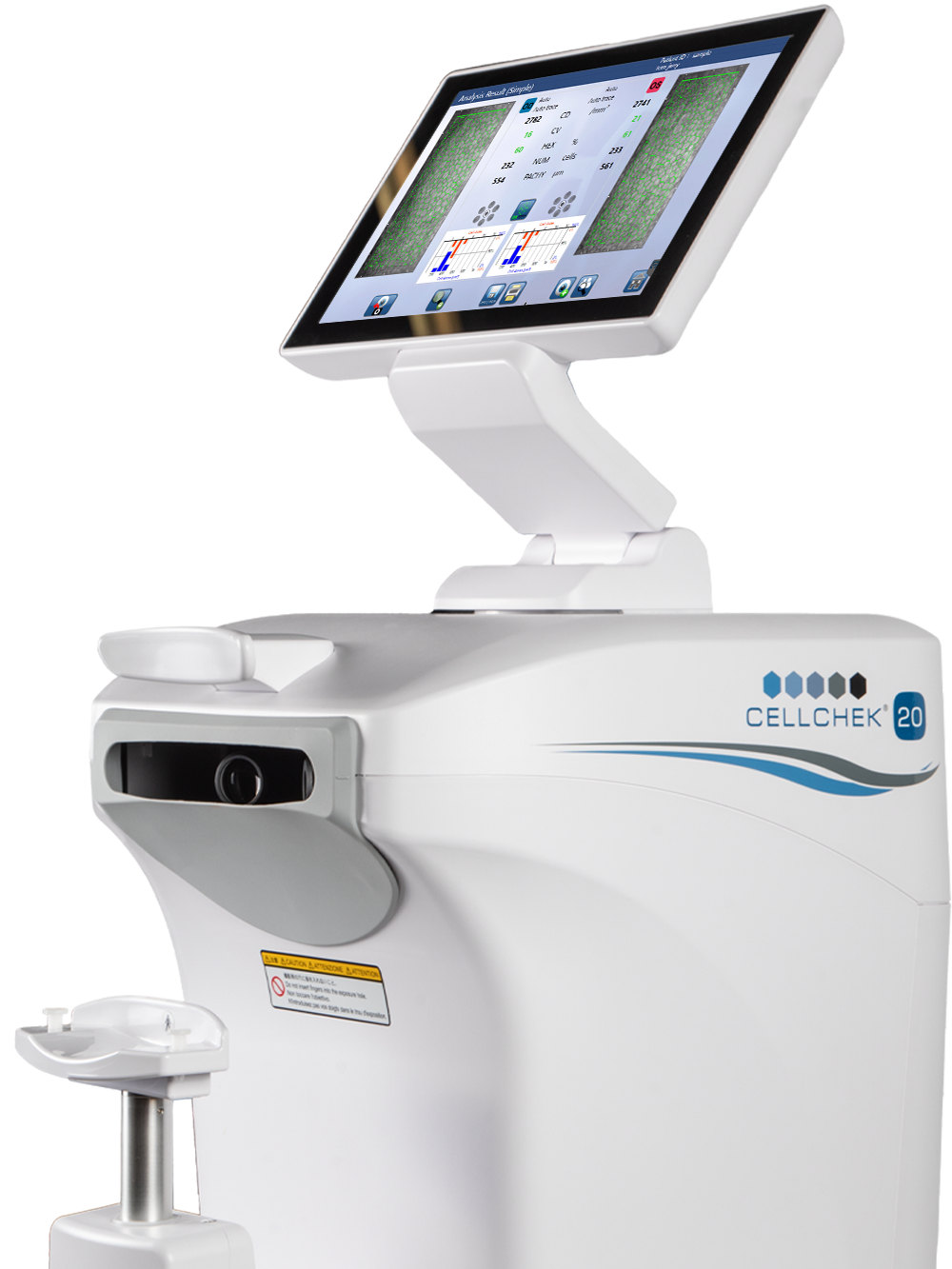
Konan Medical (Japan) and Konan Medical USA, Inc., global leaders in innovative diagnostic devices for the ophthalmic industry today announced the launch of the newest endothelial imaging system: CellChek 20.
Specular microscopy from Konan Medical is widely regarded as the Gold Standard for cellular imaging and analytic assessment of the corneal endothelium. CellChek 20 adds a single-button press exam that acquires images of both eyes and the automated analysis with the new, fully-automated Center Method™. CellChek 20 also features larger imaged area, superior image clarity as well as guttata recognition and redaction from cellular calculations.
“CellChek 20 offers a new level of convenience for our customers,” said Konan (Japan) CEO Tetsuji Ikegami. “Even during COVID, the interest has been particularly strong for high-tech, low-touch clinical specular microscopy found in CellChek 20.” Konan Medical USA CEO Charles Stewart added, “CellChek 20 has immediately generated a rebound in sales from customers and distributors. New analytic tools, compact package, and blazing fast, one-button for positioning, alignment, capture and analysis, for both eyes. The best specular product Konan has ever produced.” A demonstration video of CellChek 20 can be seen at https://youtu.be/XAw5k3I3FWU
2019
EyeKinetix is a 21st Century Alternative to the 19th Century Swinging Flashlight Method Designed to Objectively Assess Pupillary Light Reflexes (RAPDs) and more.
Irvine, CA – September 6, 2019

Konan Medical today announced that EyeKinetix is FDA Listed and now available for sale with customer deliveries commencing immediately.
EyeKinetix is Konan's 2nd generation pupillograph which is smaller, faster, easier to use, less expensive, and more full featured than its predecessor, RAPDx®, a device that brought key pupillary testing advancements, not seen for more than a century.
2018
objectiveFIELD, a visual-field testing device that requires no manual patient push-button responses, has been acquired by Konan Medical USA.
Irvine, CA – October 10, 2018
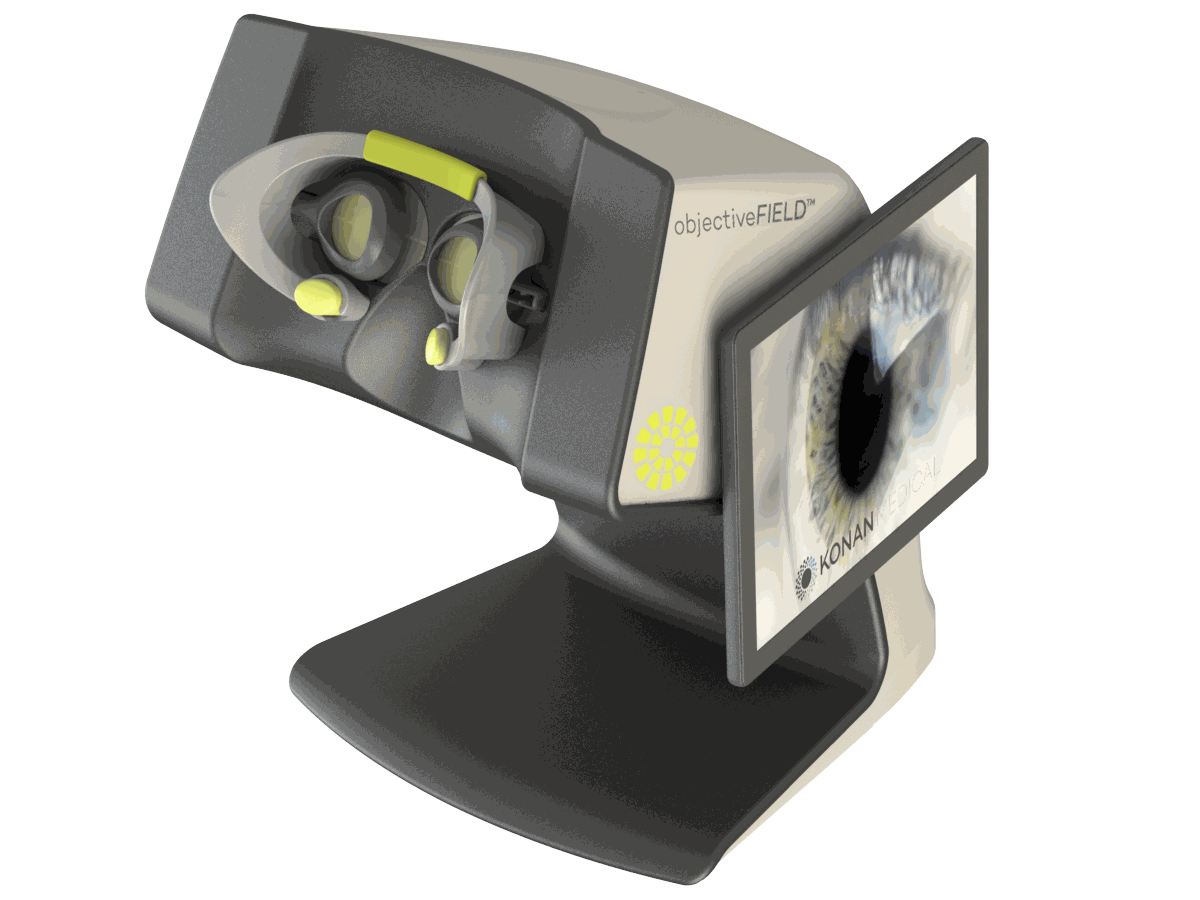
objectiveFIELD is a novel, FDA-cleared device designed to assess the human visual field in a completely objective and non-contact manner. Unlike the conventional method currently in use in most eye doctors’ offices, there is no need for any manual input from the patient. Using a patented multi-focal pupil objective perimetry method (mfPOP), objectiveFIELD tests both eyes simultaneously in only a few minutes.
objectiveFIELD has been clinically researched in Australia over more than a 12-year period with more than 16,000 examinations…
2017
Robust, User-Friendly and Affordable In-office Visual Pathway Diagnostics Technology Featuring icVEP™ Provides Important Information About the Functional Integrity of the Visual System.
Irvine, CA – July 17, 2017
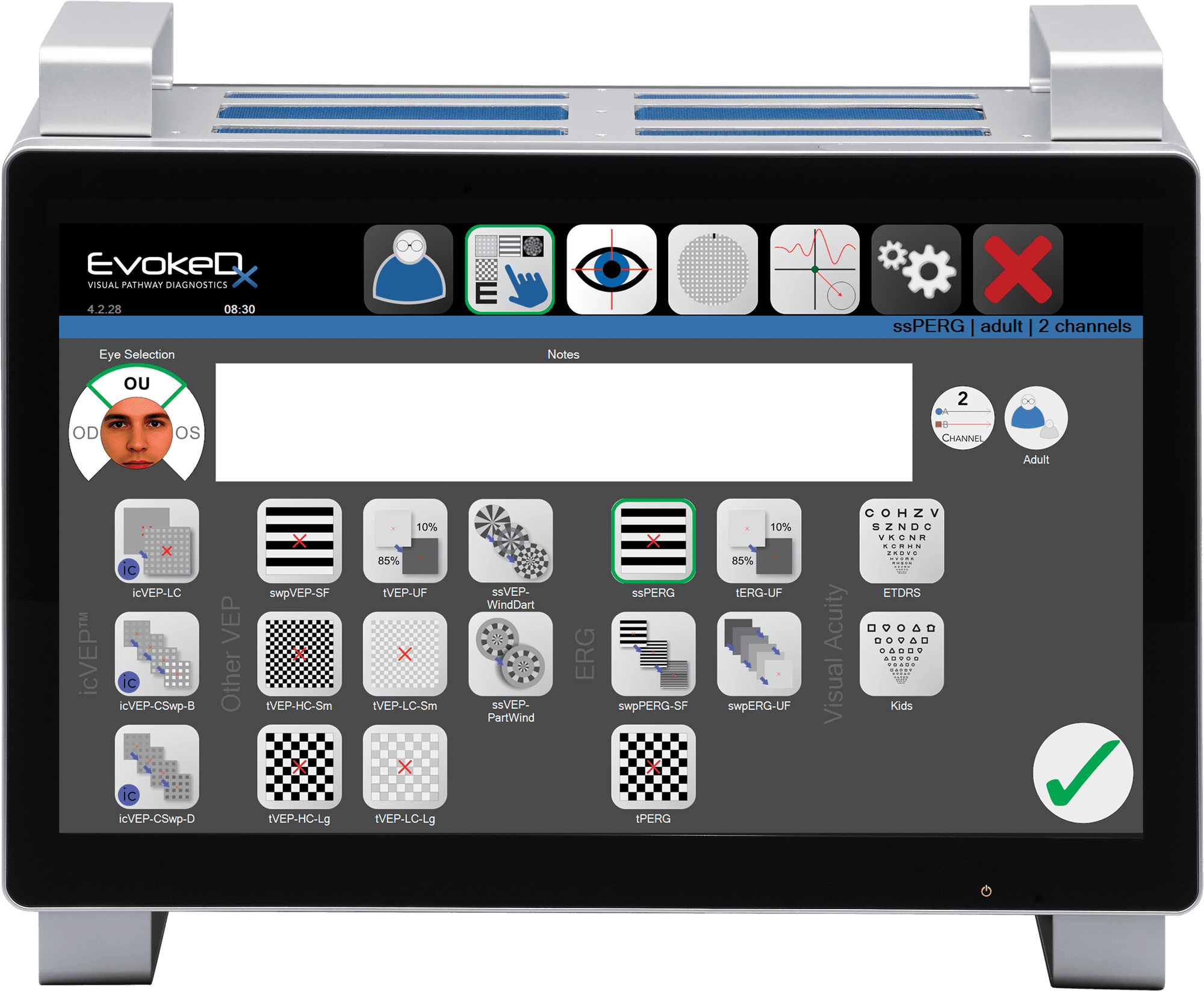
Konan Medical, a global leader in innovative diagnostic devices for the ophthalmic industry, today announced that it has attained the CE mark for its newest device, EvokeDx. The CE mark is required in Europe by 30 countries in order to market medical devices and is an assurance that the product complies with relevant European health, safety and environment protections. EvokeDx is now approved in Europe for objective assessment of central vision function including the diagnosis of glaucoma.
2015
Military Grade Extended Color Vision Testing App for iPad is now available from iTunes® and the Apple® App Store.
Irvine, CA – January 21, 2015
Konan Medical, a global leader in innovative diagnostic and examination devices for the ophthalmic industry today announced that it has released ColorDx Pro, a pseudo-isochromatic color vision test app for iPad.
2014
Robust, User-Friendly and Affordable In-office Visual Pathway Diagnostics Technology Featuring icVEP™ Provides Important Information About the Functional Integrity of the Visual System.
Irvine, CA – November 14, 2014

Konan Medical, a global leader in innovative diagnostic devices for the ophthalmic industry today announced that it has launched its newest device, EvokeDx – the next-generation visual pathway diagnostics platform to assess visual evoked potentials (VEP) and electroretinograms (ERG).
Konan Medical adds the professional-grade FLEX™ visual acuity app to the Apple iTunes store and showcased at the ASCRS meeting in Boston.
Irvine, CA – April 23, 2014
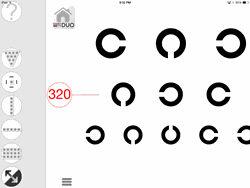
A feature-rich FLEX visual acuity app for Ophthalmology, Optometry, clinical trials and other medical uses has just been released on Apple iTunes for Retina display iPad devices as an add-in to Konan Medical’s Chart2020 Duo app. The FLEX visual acuity test allows clinicians to dynamically and instantly adjust visual acuity test parameters to test from 33 cm (14 inches) to 6 meters (20 feet). The test includes industry standard, Sloan specification literate, illiterate, and pediatric optotypes for western and 6 global languages. FLEX is gesture- driven to simply and intuitively display acuity lines, single letters, and full charts including smart randomization and reverse contrast on retina display iPad devices.
Konan Medical USA announces acquisition of rights to market Neucodia’s enhanced visual electrophysiological technology, EvokeDx™ for assessment of visual pathway disorders internationally. Featuring the patented icVEP test strategy, EvokeDx sets a new standard for clinical excellence and ease of use, at a price point that is affordable for all eye care practices.
Irvine, CA – April 08, 2014

The next generation visual evoked potential device, EvokeDx™ will be brought to market by Konan Medical USA. Visual evoked potentials have been used to assess various problems in the vision pathway (retina to brain), but have had limited appeal to Ophthalmology and Optometry practices due to difficulties in interpretation and administration. As a fully integrated system, EvokeDx sets a new standard for the eye care practice: faster, easier to use and interpret, smaller, and less expensive.
EvokeDx, a FDA 510(k) cleared system, features a patented visual stimulus “icVEP” and novel analysis methods Tcirc2 and Fstat to elicit enhanced results with simplified interpretation. Results of a recent US multi-center clinical trial were presented at ARVO in which the EvokeDx icVEP technology was used to assess glaucoma damage to the visual pathway. The study data showed that the icVEP strategy provided strong differentiation between normal eyes and glaucoma affected eyes with an overall accuracy estimate (glaucoma cases vs. controls) of A′ = 89.2%. An additional, independent study last year in Beijing, China on the technology both confirmed this accuracy of classification (92%) and provided data which led to issuance of the first VEP glaucoma diagnostic indication in China.
Konan Medical, a global leader in ophthalmic diagnostic products, has just released specialized eye medical illustrations and videos to enable improved physician – patient communication. The Media Library feature is now available on the Apple iTunes® store, and will be showcased at the Vision Expo East conference in New York.
Irvine, CA – March 19, 2014

A new Media Library has been released on Apple’s Retina iPad® as an enhancement of Konan’s Chart2020 DUO application. As with the Blind Spot Amsler™ feature, the Media Library is available for a limited time at an introductory price from the iTunes Store®. The first release of the Media Library includes patient-friendly illustrations and videos on a wide range of eye disease and surgery topics such as macular degeneration, refractive surgery, cataract surgery, color vision / color blindness, diabetic retinopathy, endothelial dystrophies and more. The Media Library was developed to be extensible with future versions allowing third party images and other educational features.
 Konan’s Amsler test is now available on Apple iTunes® store, and will be showcased at the Vision Expo East conference in New York and SECO in Atlanta.
Konan’s Amsler test is now available on Apple iTunes® store, and will be showcased at the Vision Expo East conference in New York and SECO in Atlanta.
Irvine, CA – March 13, 2014
The Amsler test for iPad® is a newly released add-in feature of Konan’s Chart2020 DUO, available for a limited time at an introductory price from the iTunes Store®. The Amsler test provides a basis for easily controlling test distances, important for repeated tests over time. The Amsler test is featured by the Macular Degeneration Foundation’s web site. Macular Degeneration is the leading cause of vision loss in Americans over age 60.
2013
Captain Mathew Rings, MC, USN has been named as the first recipient of the Aerospace Medical Association’s newest award for excellence, the Thomas J. and Margaret D. Tredici Award for his research on Konan ColorDx color vision testing and qualifications for US Navy and Marine Corps aviation.
Irvine, CA – August
13, 2013

The Aerospace Medical Association (“AsMA”) has granted the first Thomas J. & Margaraet D. Tredici Award for the most significant contribution to aerospace ophthalmology and vision science to Matthew Rings, MD. Dr. Rings was recognized for his leadership in the study of operational color vision testing and qualifications of U.S. Navy and Marine Corps aviation. Dr. Rings has done extensive research using ColorDx (Konan Medical USA, Irvine, CA) for assessment of Naval pilots. As the lead researcher, the team compared and validated various color vision testing programs for both screening and comprehensive assessment of aviation applicants and designated personnel.
(Photo shows Tredici Award recipient (center) Matt Rings, MD)
2012

Konan Medical USA’s new RAPDx high definition pupillograph has received Europe’s CE Mark and sales will begin in Europe and other countries recognizing the CE Mark beginning at the largest European Ophthalmic congress, the ESCRS, in Milan, Italy.
Irvine, CA – September
12, 2012
Konan Medical USA, Inc. has announced that its RAPDx® pupillograph for screening of defects in pupil function has received the European CE Mark. The CE Mark identifies that the RAPDx device has met the EU’s health and safety standards and the company will begin shipping product to Europe and other countries recognizing the CE Mark in conjunction with the September 2012 ESCRS meeting in Milan, Italy.
2011
Konan Medical USA’s new RAPDx high definition pupillograph has received Europe’s CE Mark and sales will begin in Europe and other countries recognizing the CE Mark beginning at the largest European Ophthalmic congress, the ESCRS, in Milan, Italy.
Irvine, CA – December 16
, 2011
Konan Medical USA, Inc. has announced that its RAPDx® pupillograph for screening of defects in pupil function has received the European CE Mark. The CE Mark identifies that the RAPDx device has met the EU’s health and safety standards and the company will begin shipping product to Europe and other countries recognizing the CE Mark in conjunction with the September 2012 ESCRS meeting in Milan, Italy.
