Mission & History of Konan
Medical

People | vision | inspiration | innovation | dedication
The Beginnings of Konan Medical
1947
Konan Medical Inc. in Japan (“KMI”), was established in 1947 in Hyogo Japan after WWII by a group of camera enthusiasts.
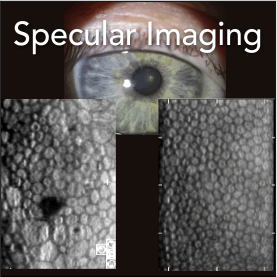
KMI has been in the specular microscope business since 1979 and holds over 1,600 patents including many remarkable products such as the balloon camera (meteorology) 1955, radar camera 1956, electrofax copy machine 1960, the first disposable camera 1963, and the specular microscope 1979.
As the market leader in specular microscopy, Konan has a significant share of the healthy and growing market for devices used to image and assess the corneal endothelium both for clinical and donor corneas / eye banking.
Constant innovation of a rock-solid product is the foundation for its well-earned leadership position in specular microscopy and fierce customer loyalty over years.

Konan's Focus
With a focus on innovative diagnostic devices, Konan Medical's flagship specular microscopes are globally recognized by eye care professionals, regulatory groups, professional reading centers, and clinical trials experts as the "Gold Standard" for endothelial cell density and morphometric analysis.
The Company's product portfolio extends into high-precision, military-grade color vision diagnostics and contrast sensitivity testing, objective assessment of visual pathway function using VEP and ERG, objective pupillometry and objective perimetry.
Konan Medical continues to earn its solid position in the marketplace with core strengths of clinical evidence collection, imaging and eye tracking, renowned customer support, expert service, and innovative competence in engineering, manufacturing and software development.
Konan's Market Position
Konan Medical USA, Inc. (KMUI) has a global sales and distribution channel comprised of in-house, virtual and geographically distributed direct sales, seasoned sales representatives, and distributors with extensive eye care product experience. Today there are over 20,000 Konan-branded products (KMI and KMUI) in use in 60 countries worldwide and in all 50 states in the USA.
Konan Medical's clinical trials services program counts a large percentage of FDA registered manufacturers of ophthalmic implantable devices and pharmaceuticals as customers.
Additionally, Konan is the market-leader in the donor cornea tissue / eye bank specular microscopy segment.
Konan's Brands
Konan Medical owns or represents these ophthalmic brands.

Intellectual Property
Konan Medical USA, Inc has over 26 issued patents in 9 countries and 8 Pending applications. Fields of coverage include objective perimetry, pupillometry, ocular motor function, concussion-related wearables, VEP + ERG electrodes, color vision and contrast sensitivity, and other objective visual functional diagnostics.
Business Development & Investment
The Company's core activities include actively searching for promising new technologies to complement its existing products, and leveraging its medical device infrastructure including global regulatory, R&D, sales and marketing, customer service, and clinical trials support services. Explore opportunities by contacting Charles Stewart (CEO) | Dale Sadlik (COO).
WE ARE PASSIONATE ABOUT PROVIDING INNOVATION AND EXCELLENCE IN OUR PRODUCTS AND SERVICES, WITH A CORPORATE CULTURE THAT IS CREATIVE, RESPECTFUL AND HARDWORKING.
WE TREAT OUR CUSTOMERS THE WAY WE WANT TO BE TREATED OURSELVES.
Konan Medical USA, Inc.
- Founding to Present -
2007 - 2008
Konan Medical USA, Inc. ("Konan Medical") is established as a California C Corporation to manage sales, marketing, and customer support in North America for Konan Japan's ("KMI") specular microscopy products.
General Manager Setsuko Susan Oak, a pioneer in specular imaging and eye banking, lays the groundwork for the new company
2009 - 2010
The Konan Medical Team is expanded with Charles Wm. Stewart as CEO and Dale Sadlik as COO. Company initiates branding, product diversification and development of a Quality System for ISO certification and FDA manufacturing. Acquisition of ONTest Corp and key Kandel patents on pupil-assessed neuro-diagnostics. Company relocates to Irvine California the global epicenter of ophthalmic device and pharma industry.
2011
The Company's role expands to include the Americas and Western Europe for KMI's products. Konan Clinical Services launched to provide global, specialized products and services supporting ophthalmic pharmaceutical and device customers with primary safety data for sponsored clinical trials.
Konan Medical achieves ISO-13485 certification.
2012
RAPDx® objective pupillometry commercialization initiated in the Americas, Europe and Asia and CE mark attained. ColorDx® brand established for medical color vision diagnostic products.
2013
Licensing of first medical color vision products (pseudoisochromatic) are added to the Konan Medical family of diagnostic products.
ColorDx is FDA listed and CE marked.
2014
Konan Medical licenses FDA cleared Neucodia product from Professor Vance Zemon and George Hu, PhD. Product introduced commercially as EvokeDx® icVEP® visual electrophysiology diagnostics. Konan patented conductive carbon electrodes developed by the Company.
2015
EvokeDx commercialized. CellChek® SL and CellChek D+ launched. US Navy begins using ColorDx. Licensing agreement with Vulintus / UT Dallas / U Iowa pupil - ocular motor - acceleration injury technologies.
2016
Company enters into a CRADA agreement with the US Air Force, OBVA Team to develop and commercialize a next-generation, high-fidelity, cone-isolation contrast sensitivity test for assessment of genetic and acquired color vision deficiencies.
2017
Konan Medical initiates USA distribution of LKC's RETeval (hand-held ERG) and disposable electrodes to complement EvokeDx. EvokeDx CE-marked with indication for the diagnosis of glaucoma. Company grows and relocates to new Irvine, California HQ. ColorDx CCT HD commercialized as first cone-contrast test to be CE-marked and FDA listed.
2018
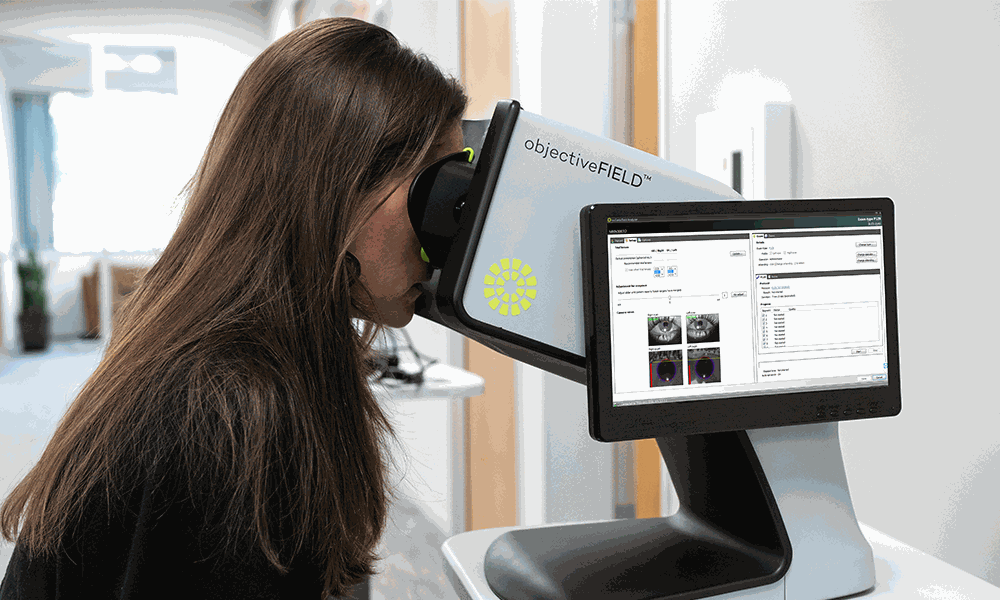
Konan acquires objectiveFIELD, a 510(k) cleared objective perimeter from Australian National University. Manufacturing engineering begins. Konan successfully completes ISO MDSAP certification audit of the Medical Device Single Audit Program, with current participation from Australia (TGA), Canada (Health Canada), and USA (FDA).
2019
EyeKinetix commercialized with initial uses (RAPDx test). New Konan Medical branding initiated including trademarked Konan Iris.
objectiveFIELD prototypes demonstrated.
2020
2020 quickly became about implementing a creative, effective response to Covid-19 and its associated fallout such as the cancellation of industry meetings. Konan's most valuable assets, our human assets, are fully retained during the pandemic.
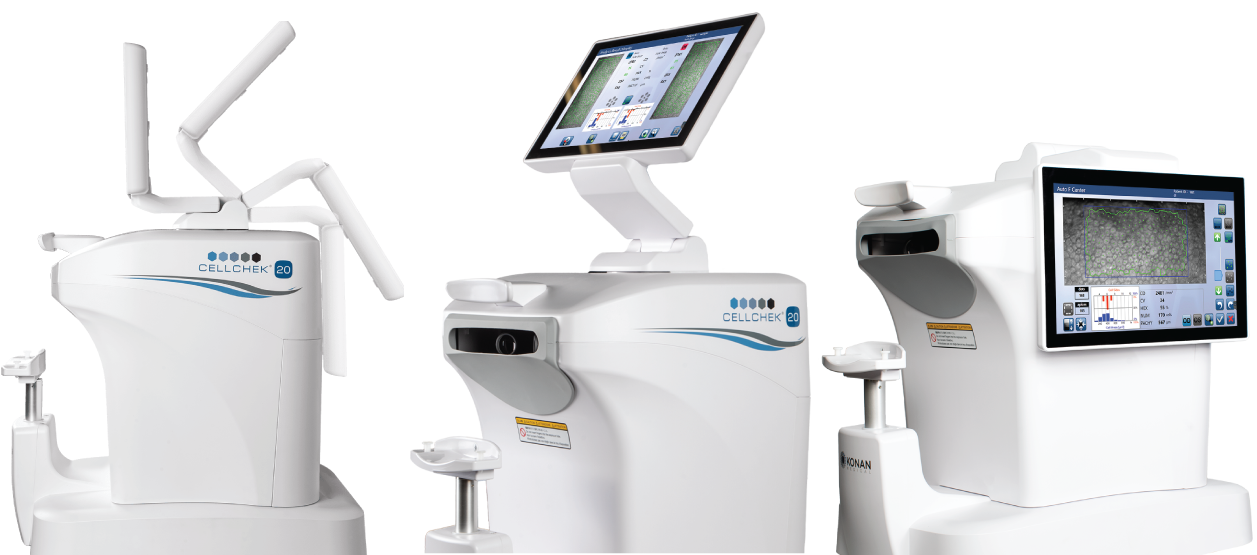
A major model change for specular imaging: CellChek 20 commercialized. One touch, blazingly fast. Continued uptake in EyeKinetix pupillography, and progress on OFA manufacturing engineering despite COVID critical supply chain disruptions.