EvokeDx®
Frequently Asked Questions &
Specifications
Frequently Asked Questions
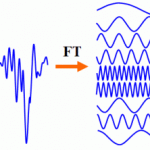 Fourier TransformTraditionally, visual electrophysiology data has been analyzed in the time domain, typically with a transient response, looking at one or two peaks to try to draw conclusions as to whether or not the response is affected. EvokeDx features a discrete Fourier transform from the time domain to the frequency domain, allowing data from the entire response to be included in the analysis.
Fourier TransformTraditionally, visual electrophysiology data has been analyzed in the time domain, typically with a transient response, looking at one or two peaks to try to draw conclusions as to whether or not the response is affected. EvokeDx features a discrete Fourier transform from the time domain to the frequency domain, allowing data from the entire response to be included in the analysis.
Much as when OCT leaped from the time domain to the spectral domain, an entirely new set of statistical tools are available in the frequency domain to simplify the clinician’s assessment of VEP and ERG responses.
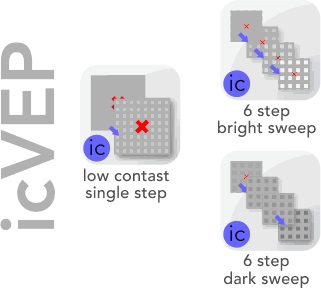 EvokeDx is designed for visual electrophysiology measurement. This includes VEP, ERG, and our proprietary icVEP test strategy that was the subject of an NIH funded, multi-center clinical trial. icVEP is a low-contrast, high temporal frequency pattern that sinusoidally varies in contrast at 10 Hz. With a well functioning pathway, and the Fourier Transform, we expect to measure a sinusoidal variance in signal at exactly 10 Hz at the visual cortex.
EvokeDx is designed for visual electrophysiology measurement. This includes VEP, ERG, and our proprietary icVEP test strategy that was the subject of an NIH funded, multi-center clinical trial. icVEP is a low-contrast, high temporal frequency pattern that sinusoidally varies in contrast at 10 Hz. With a well functioning pathway, and the Fourier Transform, we expect to measure a sinusoidal variance in signal at exactly 10 Hz at the visual cortex.
This novel test strategy is available in three variants: single contrast bright check, sweep of contrasting, bright checks, and sweep of contrasting dark checks.
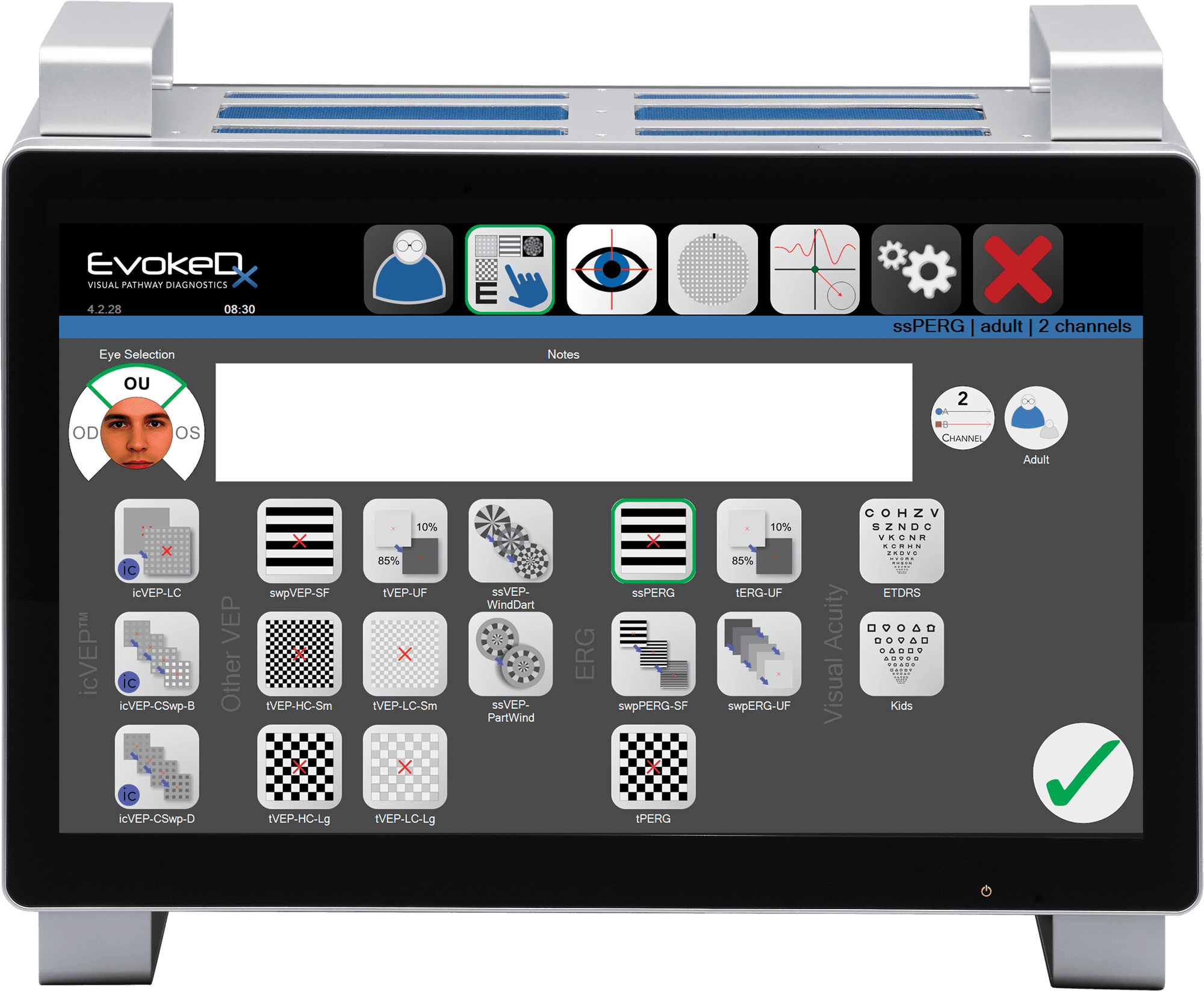
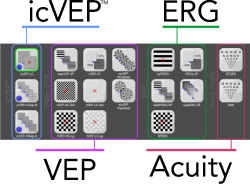 EvokeDx libraryEvokeDx features multiple visual electrophysiology test configurations with commonly used parameters for icVEP, VEP, and ERG.
EvokeDx libraryEvokeDx features multiple visual electrophysiology test configurations with commonly used parameters for icVEP, VEP, and ERG.
Visual electrophysiology has been performed for decades using a large assortment of EEG sensors, both generic and speciality, built to gather as large as possible amplitudes in the least invasive manner (something that is generally mutually exclusive). Konan strategically utilizes standard snap connectors to give our customers a choice, both now and in the future, of the types of electrodes selected. Use of a proprietary connector would have locked you into purchasing single-supplier, high-cost products.
The standard EEG skin electrodes used for ERG testing are easy to prep for, apply, and also provide good signal amplitudes.
![]() Our gaze monitoring system provides an on-screen video feed of the patient for the technician to easily monitor test progress and patient attention. Inattention, excessive blinking, and closed eyes will distort the collected data. Prompt attention to attentiveness will mean fewer test repeats, happier patients and better office efficiency.
Our gaze monitoring system provides an on-screen video feed of the patient for the technician to easily monitor test progress and patient attention. Inattention, excessive blinking, and closed eyes will distort the collected data. Prompt attention to attentiveness will mean fewer test repeats, happier patients and better office efficiency.



![]()
Features
| FEATURE | BENEFIT |
|---|---|
|
OLED stimulus display
|
Premium specification, with linearization and microsecond timing, successfully addresses luminance artifact contamination known to affect LCD displays
|
|
NIH clinical data
|
Multi-center clinical study: differentiation of glaucoma and normals with icVEP-LC
1
|
|
All-in-one design
|
Beautiful, compact, delivered in one box, and can be mobilized with optional case
|
|
Patented icVEP test strategies
|
Low Contrast Bright (single step), Bright Sweep, Dark Sweep
|
|
Gaze monitor
|
On-screen gaze monitoring allows operator to easily monitor both test recording and patient attention |
|
Test library
|
16 different icVEP, VEP, ERG
|
|
EvokeDx Viewer
|
Viewer application allows secure remote access by physician to test results on device
|
|
Touch-screen UI
|
Double-shielded, internal amplifier ... no need for the patient to hold parts of the test equipment
|
|
Fourier transform analytics
|
T2circ, Sine : Cosine, Amp-Phase, and MSC
|
|
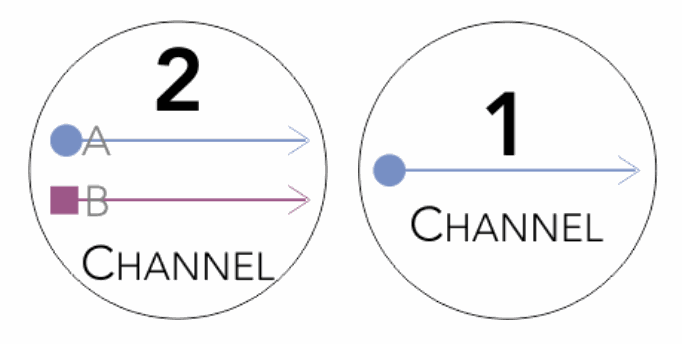
Single or dual channel recording
|
Record single channel (3 lead) or bilateral ERG or simultaneous VEP & ERG (5 lead) ... in one go!
|
|
Very short interval testing
|
Test is divided into multiple short sequences typically 2 to 6 seconds each
|
|
Luminance calibration
|
Simple (auto and semi-auto) calibration, linearization, and calibration history
|
|
Child-attention animations
|
Selectable high-quality videos to capture and hold attention
|
|
Synchronous data collection
|
Data collection is synchronized using low-latency Polling technique (non-IRQ) with the stimulus frame rate to eliminate time gap shifting and enhance Fourier transformed calculations
|
|
Auditory cues for patient
|
No sound (or associated data contamination) from intra-test sound files
|
|
Low-cost, disposable electrodes
|
Other systems may require costly, proprietary electrodes ... EvokeDx uses low-cost solutions for patient disposables.
|
| EMR | EHR |
Local or network reports configuration
|
| Simple software upgrades |
Software is easily kept current with either on-request or on-screen notice of a new version
|
|
Integrated data backup
|
Local or network locations
|
Specifications
| EVOKEDX STIMULUS | |||
|---|---|---|---|
|
Resolution
|
1920 x 1080
|
Patterns
|
Patented isolated-check, checkerboard, gratings, windmill-dartboard, uniform field
|
|
Frame rate
|
60 Hz
|
Temporal functions
|
Sine wave, square wave, superimposed two sinusoid
|
|
Grey level
|
8 bit resolution
|
Sweep display
|
Up to 10 steps with variable contrast, spatial frequency, and
temporal frequency |
|
Gamma correction
|
Software
|
|
|
|
DATA ACQUISITION
|
|||
|
Analog - digital
conversion |
16 bit resolution
|
Data-timing control
|
Low-latency Polling method (non-IRQ) to synchronize data collection to stimulus frame rate to decrease variability in response measures
|
|
Sampling rate
|
480 S/s
|
Electrodes
|
3 and 5 with disposable AgCl
|
|
SIGNAL AMPLIFICATION - meets or exceeds ISCEV - International Society for Clinical Electrophysiology of Vision and FDA regulatory requirements
|
|||
|
Channels
|
Differential, One or Two
|
Filters
|
Fourier bandwidth 0.5 to 100 Hz, Fourier notch filter
|
|
Gain
|
20,000
|
Input range
|
+/- 6 mV
|
|
CMRR
|
Common Mode Rejection
Ratio > 120 dB |
Input impedance
|
2 X 1012
|
| Ohm isolation voltage | 1.5 kV |
Power supply
|
+8 to +15 VDC |
|
DATA PROCESSING
|
|||
|
Steady-state response
|
Spectrum analysis
|
T
2
CIRC
|
Digital Fourier Transform driven statistical analysis reducing complex waveform to amplitude and phase angle
|
|
Transient response
|
Artifact removal
|
MSC
|
Coherence (Magnitude Squared Coherence) of recorded response
compared to frequency response noise |
|
Digital filters
|
Low, high, even/odd, notch
|
FSTAT
|
Statistical definition of difference between two samples and confidence interval
|
|
SYSTEM HARDWARE
|
|||
|
Integrated computer
|
Operator: 22" touchscreen
|
Power
|
100-240 VAC; 50/60 Hz; 5.5 A; isolation transformer included
|
|
Gaze / eye monitoring
|
Infrared 770-950 nm camera (ISO grp)
|
WiFi / bluetooth
|
Printing, EMR network communications, secure support
|
|
Dimensions
|
20.7 x 9.5 x 16.4 inches W x D x H (52.6 x 41.6 x 16.2 cm)
|
Weight
|
50 lbs (22.7 kgs)
|
|
REGULATORY
|
|||
|
USA
|
FDA 510(k) K081591
5
|
Japan
|
Certificate # 229AGBZX00100000 (COSMOS CORP)
|
|
Europe
|
CE mark
|
|
|
1. US Patent 06966650: Method and Apparatus for and Automated Procedure to Detect and Monitor Early-stage Glaucoma. 2. Victor JD, Mask J. A new statistic for steady-state evoked potentials, Electroencephalogy Clin Neurophysiol, 1991, 78:378-388. 3. Zemon V, Tsai JC, et al. Novel electrophysiological instrument for rapid and objective assessment of magnocellular deficits associated with glaucoma, Doc. Ophthalmol, 2008, 17:233-243. 4. Kim D, Zemon V, Saperstein A, Butler B, Javitt D. Dysfunction of early-stage processing in schizophrenia: harmonic analysis, Schizophr. Res, 2005, 76:55-65. 5. FDA 510(k) K081591. This device is used as a tool for assessment of visual function. It cannot be used as a definitive diagnostic indicator. The configuration and interpretation of the test shall be made by users based upon their knowledge and understanding of VEPs in response to stimuli. Diagnosis of a patient is the responsibility of a licensed physician.
